Determine the Oxidation Number of Each Element in if
Ans-A In CaF2 the oxidation number of Ca is 2 that of F is -1. Based on the assumption that there are 100 grams of the unknown substance you can determine that the number of grams present for each element equals the percentage value of each element mentioned in the problem.

Oxidation Number How To Find Oxidation State
While fully ionic bonds are not found in nature many bonds exhibit strong ionicity making.

. In each of those three cases you can determine the oxidation state of manganese by using the known oxidation state of oxygen and the overall charge of the ion when that is the case. Determine which atoms are oxidized and which are reduced. Since the magnesium atom has a 2 oxidation number this means that each chlorine atom must have a -1 oxidation number.
If the group is of d or f type an amount of 100 for. Ununennium also known as eka-francium or element 119 is the hypothetical chemical element with symbol Uue and atomic number 119. Oxidation number is a number that is assigned to an element in a.
Ill show you how to find manganeses oxidation state in the first two compounds and leave the last one to you as practice. In the periodic table of the elements it is expected to. -2 2 0.
Earth-abundant element based electrocatalysts for electrochemical alcohol oxidation have been studied to potentially replace the expensive and rare noble metal-based catalysts. Charge on each ligand one can determine the oxidation number for the metal. The simplest way to interpret oxidation number is to think of it as the number of electrons lost or gained by an atom compared to its neutral uncombined form when it reacts to form ions or molecules.
Atomic number9 atomic weight18998403163 melting point21962 C 36332 F. Ans-B In H2SO4 the oxidation number of H is 1 that of S is 6 that of O is -2. Oxidation state or oxidation number is a bookkeeping device employed by chemists to help them classify and understand chemical reactions.
Oxidation Numbers Or. There is a change in the oxidation number of the other carbon atom however from -1 to 3. Its chemical activity can be attributed to its extreme ability to attract electrons it is the most electronegative element and to the small size of its atoms.
Ans-D In HF the oxidation number of H is 1 that of F is -1. Emission element line spectra. Each nuclide is denoted by chemical symbol of the element this specifies Z with tha atomic mass number as supescript.
If the group n is of s p type an amount of 085 from each electron in n-1th shell and an amount of 100 for each electron from n-2 and lower shells is added to the shielding constant. The number of atoms of each element on both sides of the equation is the same and therefore mass is conserved. 2 chlorine atoms give us a total of -2.
Therefore we cannot determine the neutron number of uranium for example. The oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively which are used until the element is discovered confirmed and a permanent name is decided upon.
We can determine the neutron number of certain isotope. Heres what you have here. Fluorine F most reactive chemical element and the lightest member of the halogen elements or Group 17 Group VIIa of the periodic table.
Ans-C In CaSO4 the oxidation number of Ca is 2 that of S is 6 that of O is -2. For example the neutron number of uranium-238 is 238-92146. Among them Ni-based catalysts have received increased attention due to their competitive price high stability in alkaline media low poisoning effect and high catalytic activity toward various alcohol.
Determine the number of grams for each element. Letting y be the oxidation number of phosphorus -1 y 21 4-2 y oxidation number of P 5. The oxidation number of the metal is given by a Roman numeral in parentheses after the metal.
Identifying Reduced and Oxidized Elements Determine which element is oxidized and which element is reduced in the following reactions be sure to include the oxidation state of each.
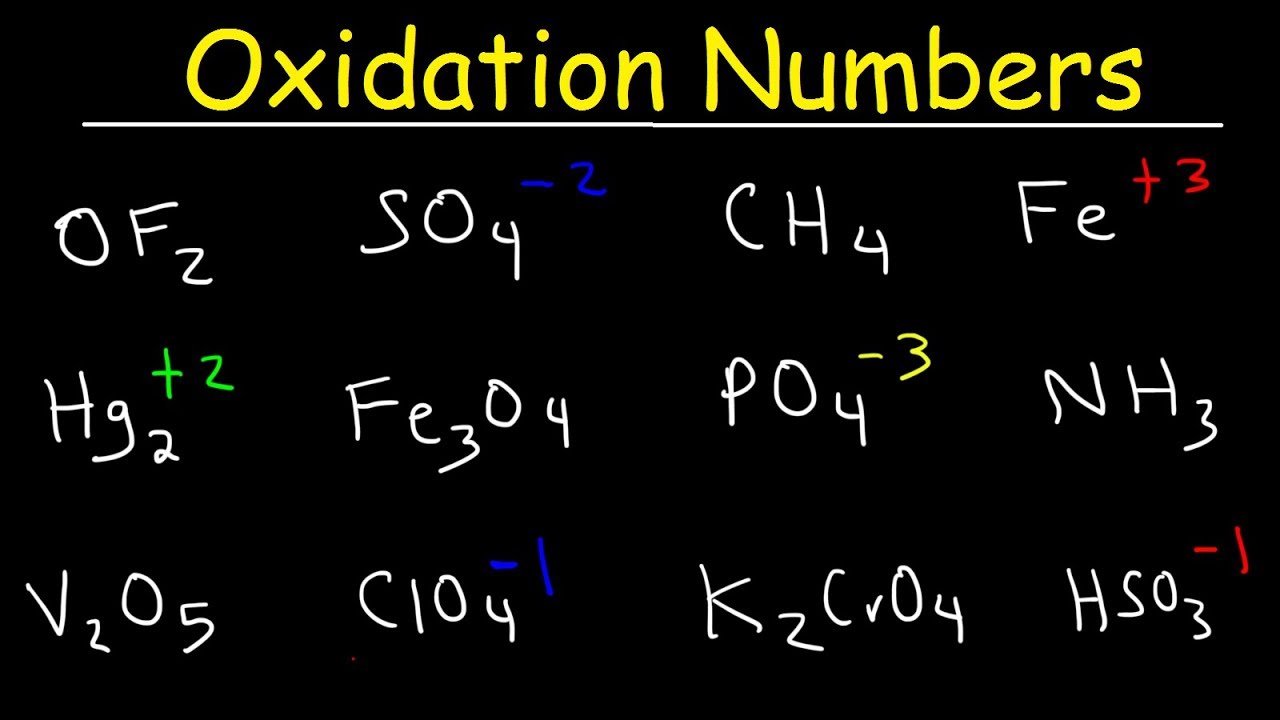
How To Calculate Oxidation Numbers Basic Introduction Youtube

Assign Oxidation Number To The Underlined Elements In Each Of The Following Species A Nah 2 P Youtube
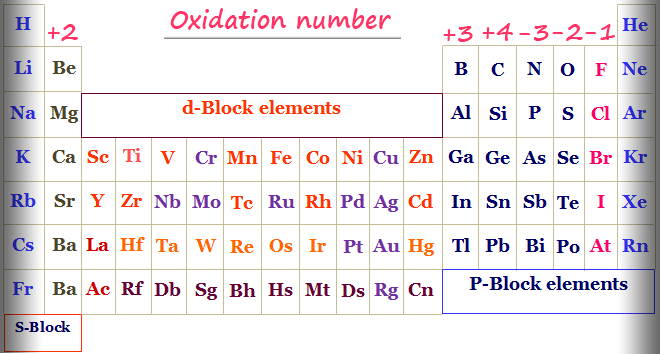
Belum ada Komentar untuk "Determine the Oxidation Number of Each Element in if"
Posting Komentar